

Just like how you'd rather sit in a comfy chair than a hard one, electrons prefer to be in a lower energy state. When it comes to filling up orbitals with electrons, they follow a pretty simple rule: they always go for the lowest energy level first. We’ll take a look at both of them in turn before putting them into practice with some examples. These are known as Hund’s rule and the Aufbau principle. There are two main rules that you should know that will help you work out an atom’s electronic configuration. So, electron configuration is really just a way of saying how many electrons are in each orbital, and which shell and sub-shell they're in. The shape of the orbital depends on which sub-shell it's in. It's like a tiny electron clubhouse! Each orbital can only have two electrons and they have to have different spins. Orbitals are like tiny spaces where electrons hang out. The different energy levels of shells, subshells and orbitals For example, the s sub-shell has just one orbital whilst p sub-shells have three and d sub-shells have five. Each sub-shell contains different numbers of orbitals. They also have different energy levels - the s sub-shell has the lowest energy, then p, then d, then f. Sub-shells are divisions within each shell. As shells get further from the nucleus, their principal quantum number increases and they have a higher energy level. Each shell has a specific principal quantum number. Electron shellsĮlectron shells are also known as energy levels. For now, we’ll just provide a quick summary. If you’re not familiar with the above terms, we recommend looking at Electron Shells to learn a bit more about them. What is electron configuration?Įlectron configuration, also known as electronic configuration, is the arrangement of electrons in shells, sub-shells, and orbitals within the atom. Finally, we'll look at the evidence that supports electron configuration.
#Carbon electron configuration standard form how to
We'll also work through some examples to help you understand how to calculate electron configuration. We'll define it, look at how it's represented, and learn about the Aufbau principle and Hund's rule. In this article, we'll explore the basics of electron configuration. But how do we figure out the electron configuration of an element or ion? It's an important concept in physical chemistry and helps us understand the reactivity and chemical properties of an element.
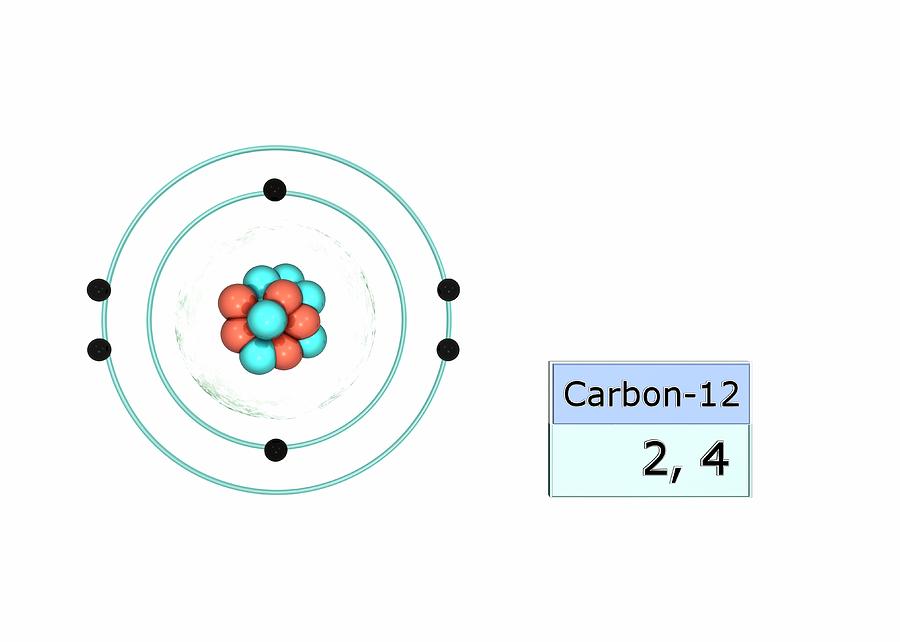
Those tiny little subatomic particles, whizzing around in their orbits.Electron configuration is a way of describing the arrangement of electrons in an atom or ion. Saturated Unsaturated and SupersaturatedĪh, electrons.Reaction Quotient and Le Chatelier's Principle.

Prediction of Element Properties Based on Periodic Trends.Molecular Structures of Acids and Bases.Ion and Atom Photoelectron Spectroscopy.Elemental Composition of Pure Substances.Application of Le Chatelier's Principle.Structure, Composition & Properties of Metals and Alloys.Intramolecular Force and Potential Energy.Variable Oxidation State of Transition Elements.Transition Metal Ions in Aqueous Solution.Single and Double Replacement Reactions.


 0 kommentar(er)
0 kommentar(er)
